Acid and Base Chart: Quick Guide to Strength and Reactivity
Description
Let's have a quick guide about the acid and base chart. The guide explains the role of acids, bases, and salts in everyday science and industrial practices.
Acids, Bases, and Salts
Acids have a sour taste. They can burn or irritate the skin. For instance, vinegar and lemon juice are common acids. Bases have a bitter taste. They feel slippery. Soap is a common base that many people have used. Salts are left behind when acids and bases react. Table salt is a common salt. In simple language, the chart shows how strong different acids and bases are. It also shows how they react with each other. The guide uses common examples to help show these points.
Summary Table: Strength of Acids and Bases
|
Acids/Bases |
Formula |
Approx. pKa |
Notes |
|
Strong Acids |
|||
|
Hydrochloric acid |
HCl |
–7 |
Strong mineral acid |
|
Sulfuric acid (1st H) |
H₂SO₄ |
–9 |
Diprotic, only 1st dissociation strong |
|
Nitric acid |
HNO₃ |
–1.4 |
Strong oxidizing acid |
|
Hydrobromic acid |
HBr |
–9 |
Strong acid |
|
Hydroiodic acid |
HI |
–10 |
Strong acid |
|
Perchloric acid |
HClO₄ |
–10 |
One of the strongest acids |
|
Weak Acids |
|||
|
Acetic acid |
CH₃COOH |
4.76 |
Found in vinegar |
|
Carbonic acid |
H₂CO₃ |
6.35 (1st) |
Forms in CO₂-water equilibrium |
|
Hydrofluoric acid |
HF |
3.17 |
Weak, but highly corrosive |
|
Phosphoric acid |
H₃PO₄ |
2.15 (1st) |
Triprotic acid |
|
Formic acid |
HCOOH |
3.75 |
Found in insect venom |
|
Citric acid |
C₆H₈O₇ |
~3.1 |
Organic acid, triprotic |
|
Strong Bases |
|||
|
Sodium hydroxide |
NaOH |
~0 |
Common strong base |
|
Potassium hydroxide |
KOH |
~0 |
Similar to NaOH |
|
Calcium hydroxide |
Ca(OH)₂ |
~1.4 |
Sparingly soluble |
|
Barium hydroxide |
Ba(OH)₂ |
~0.15 |
Strong, highly soluble |
|
Weak Bases |
|||
|
Ammonia |
NH₃ |
4.75 |
Common weak base |
|
Methylamine |
CH₃NH₂ |
3.36 |
Simple organic base |
|
Pyridine |
C₅H₅N |
8.75 |
Aromatic heterocyclic base |
|
Aniline |
C₆H₅NH₂ |
9.4 |
Aromatic amine, very weak base |
|
Bicarbonate ion |
HCO₃⁻ |
~7.6 |
Amphoteric, part of buffer system |
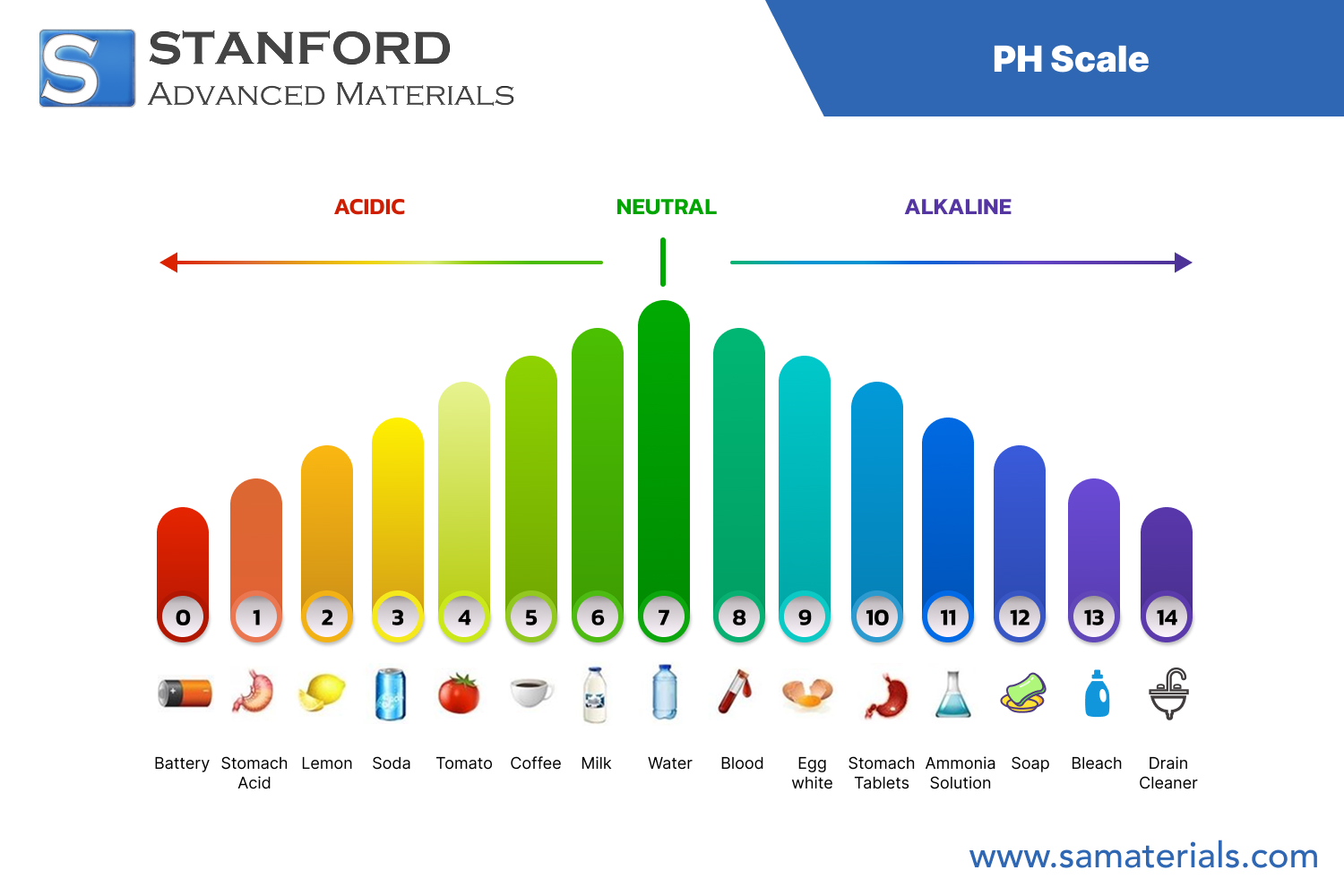
Further reading: PH Scale: Acids, Bases, and Common Materials
The table below gives a summary of common acids and bases along with their strength. The scale of strength typically ranges from very weak to very strong. Here is a glance at the content:
• Strong Acids: Examples include hydrochloric acid and nitric acid. These acids ionize completely in water. They can lead to significant changes in pH.
• Weak Acids: Examples include acetic acid and citric acid. They do not fully dissociate in water. They show mild reactivity compared to strong acids.
• Strong Bases: Common examples include sodium hydroxide and potassium hydroxide. They fully dissociate in water and produce high pH solutions.
• Weak Bases: An example is ammonium hydroxide. They only partially dissociate. They have a lower reactivity compared to strong bases.
This summary table helps in laying out the relative reactivities and strengths. Each entry is based on the percentage of ionization in an aqueous solution and the associated pH levels.
Understand Acid-Base Relationships
Acids and bases work in pairs. When they mix, they neutralize each other. The process usually results in the production of water and a salt. This is crucial in many natural processes and industrial applications. For example, the human stomach uses hydrochloric acid to break down food. In another example, bases are used to clean and neutralize spills. The chart shows the balance between acids and bases. It gives a good idea of the strength of the reaction between the two. Simple charts like this help many students and professionals to correlate pH changes with chemical reactions.
How Acid-Base Pairs Stabilize PH
Acid-base pairs are important in stabilizing pH. A buffer is a common system that uses these pairs. A buffer can hold back rapid changes in pH. In our bodies, buffers are used to stabilize the pH of blood. They keep the system in a safe range. The chart shows how acid-base pairs work together. In many chemical processes, a buffer helps handle the small increases or decreases in pH. This property is important in industrial processes that require a controlled pH. A stable pH results in more efficient chemical reactions. The data in the table is made easy to understand with real-world examples. The acid and base interactions are shown clearly and concisely.
Conclusion
The acid and base chart is a useful tool for anyone who works with chemicals. It shows the differences in strength and reactivity in a clear manner. By understanding the chart, people can make safe decisions when handling acids and bases. For more information and tech support, please check Stanford Advanced Materials (SAM).
Frequently Asked Questions
F: What does a strong acid do in water?
Q: It ionizes almost completely, producing a high concentration of hydrogen ions.
F: What is a buffer solution?
Q: It is a mixture that resists changes in pH by using acid-base pairs.
F: Why is pH stability important?
Q: It ensures safe chemical reactions and proper function of biological systems.

 Bars
Bars
 Beads & Spheres
Beads & Spheres
 Bolts & Nuts
Bolts & Nuts
 Crucibles
Crucibles
 Discs
Discs
 Fibers & Fabrics
Fibers & Fabrics
 Films
Films
 Flake
Flake
 Foams
Foams
 Foil
Foil
 Granules
Granules
 Honeycombs
Honeycombs
 Ink
Ink
 Laminate
Laminate
 Lumps
Lumps
 Meshes
Meshes
 Metallised Film
Metallised Film
 Plate
Plate
 Powders
Powders
 Rod
Rod
 Sheets
Sheets
 Single Crystals
Single Crystals
 Sputtering Target
Sputtering Target
 Tubes
Tubes
 Washer
Washer
 Wires
Wires
 Converters & Calculators
Converters & Calculators
 Write for Us
Write for Us





 Chin Trento
Chin Trento